Request further information
If you would like additional information on Hemospray contact us »
 Learn More
Learn More
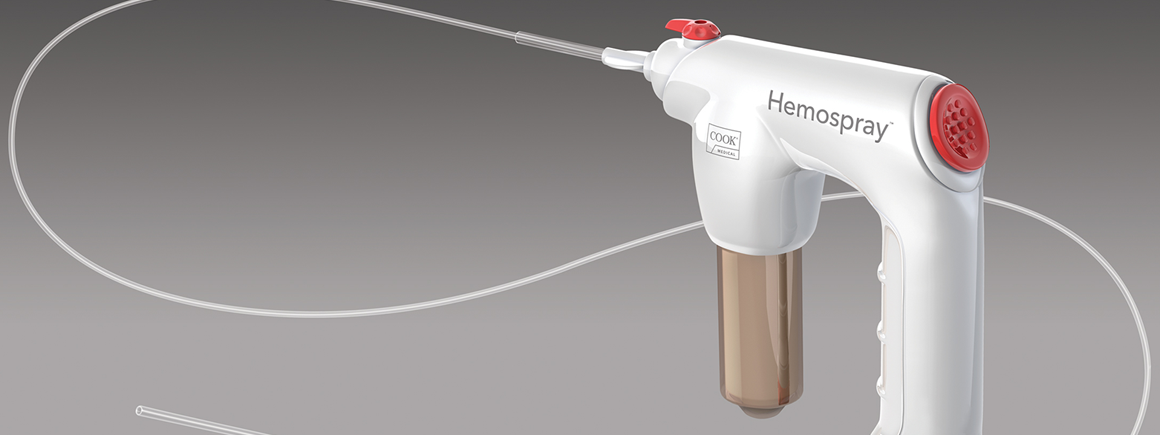
Hemospray is a proprietary mineral blend powder developed specifically for endoscopic haemostasis. It contains no human or animal proteins or botanicals and has no known allergens. Hemospray is metabolically inert and deemed nontoxic, systemically or otherwise. Over the years, similar materials have been used by the military for topical battlefield haemostasis applications.
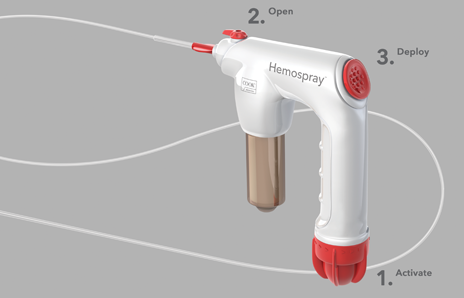
In three steps you can deploy Hemospray to treat nonvariceal GI bleeds.
1. Activate the CO2 cartridge.
2. Open the valve.
3. Spray toward the source of bleeding.
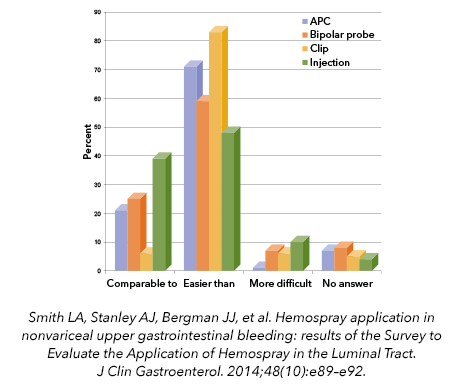
An ease-of-use comparison was made for 79 patient treatments in a postmarket clinical registry, and Hemospray was determined to be comparable to or easier than administration of other haemostasis treatment modalities.
Haemostasis was achieved in under 10 minutes in over 77% of cases with Hemospray. Hemospray was thought to save time in 59% of cases (57/97).
Contributors SEAL post market evaluation: University of Manitoba, Winnepeg MT, St. Pauls Hospital, Vancouver BC, St Michaels Hospital, Toronto ON, McGill University, Montreal QC, University Hospital Mainz, Germany, Queen Elizabeth Hospital, Birmingham, UK, Lund University Hospital, Malmo Sweden, Institute University Parc Tauli, Sabadell, Spain, Hvidovre Hospital, Copenhagen, Denmark, Hospital Cochin, Paris, France, Glasgow Royal Infirmary, Glasgow, Scotland, Erasmus Medical Center, Rotterdam, Netherlands, Ospedale San Paolo, Italy, Amsterdam Medical Center, Amsterdam Netherlands
Smith LA, Stanley AJ, Bergman JJ, et al. Hemospray application in nonvariceal upper gastrointestinal bleeding: results of the Survey to Evaluate the Application of Hemospray in the Luminal Tract. J Clin Gastroenterol. 2014;48(10):e89–e92.

‘Hemospray is an important and novel therapy which offers an exciting new treatment option in patients at highest risk from bleeding lesions in the upper GI tract.’
—John Morris, MD, Glasgow Royal Infirmary, Glasgow, Scotland
Dr John Morris was a paid consultant at the time of the study.
If you would like additional information on Hemospray contact us »